ECG Mobile Cardiac Monitoring
with MediBioSense VitalPatch
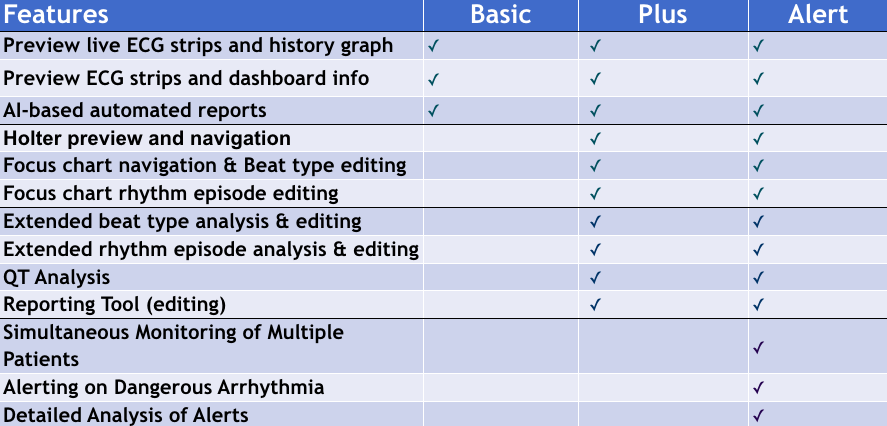
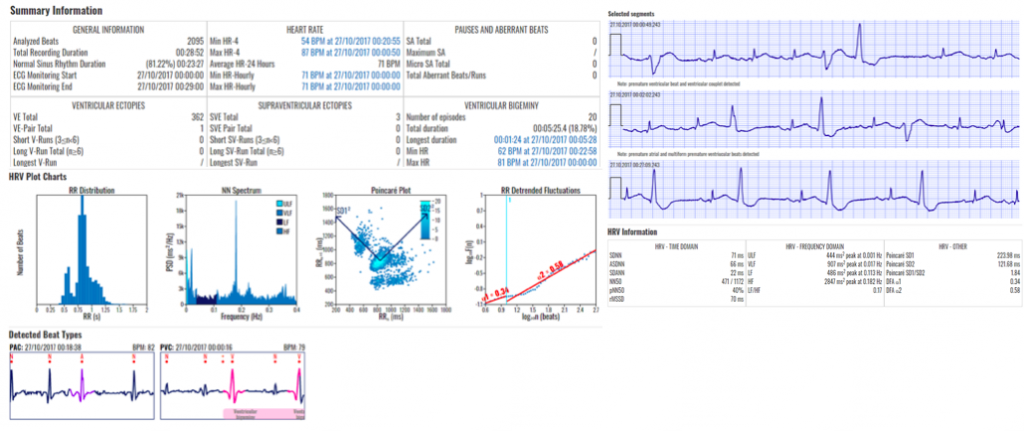
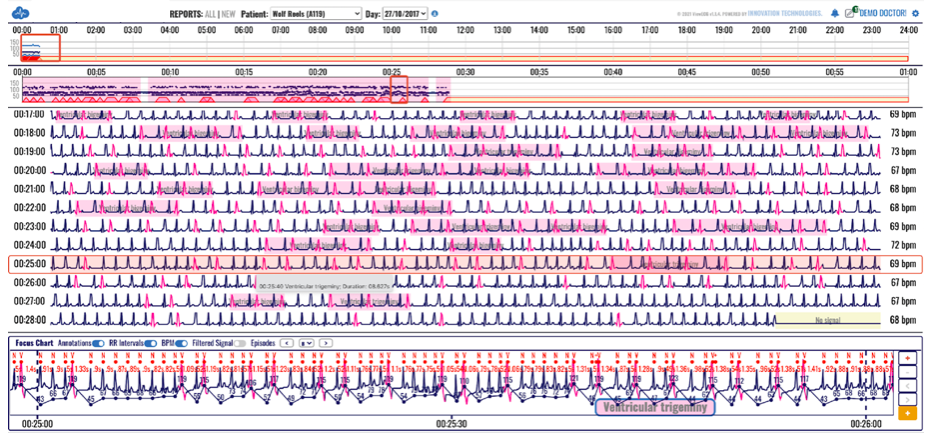
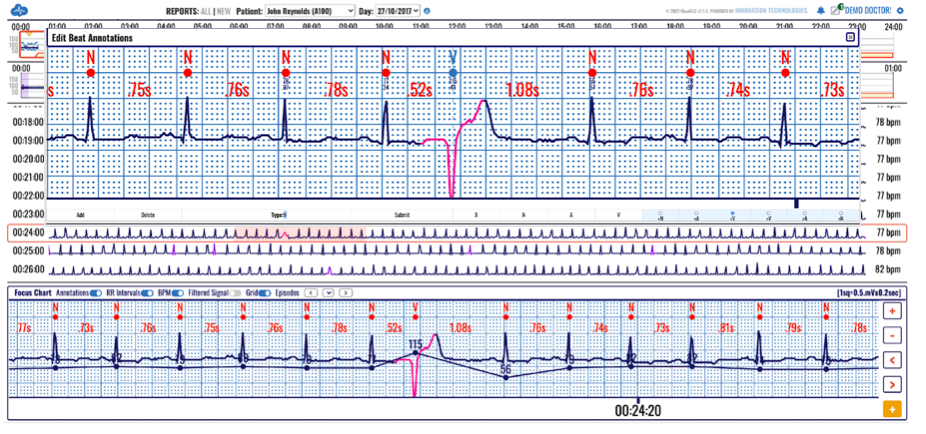
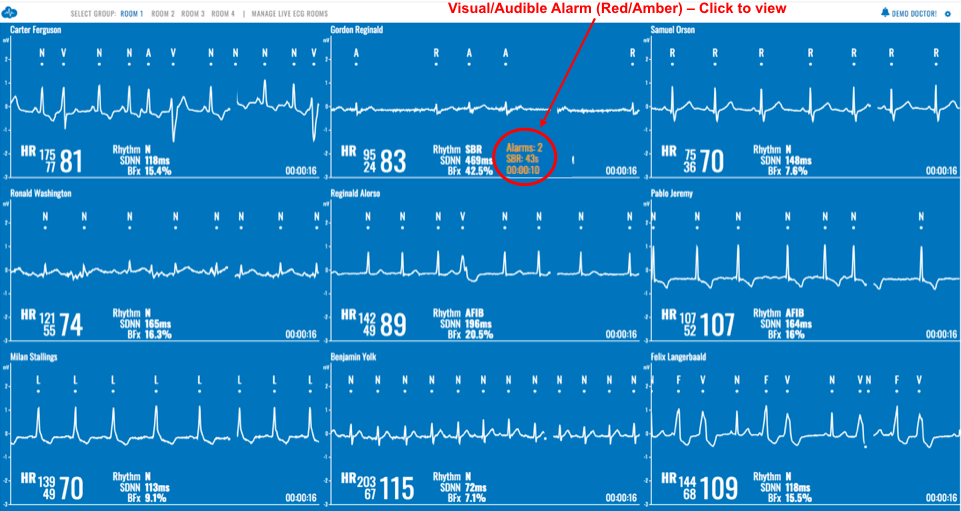
The only cardiac monitoring technology that sends patient data instantaneously to a secure cloud, enabling live remote monitoring for 21 different cardiac arrhythmias and real-time ECG alerts.